Overflowing with Antibodies and Optimism
There is more optimism than ever that researchers are on the path to developing vaccines and antibodies that can help stem HIV’s persistent spread.
“A new era. An exciting time. More optimism than ever before.” These are just some of the ways researchers are describing the current state of play in HIV vaccine and antibody research.
There is very exciting new research that gives us great hope that we are making substantial progress with vaccines and antibodies. We have better vaccine antigens in the last few years than we’ve ever had, and also better vaccine platforms. An effective vaccine is likely, and we’re on that path.
John Mascola, director of the Vaccine Research Center (VRC) at the U.S. National Institute of Allergy and Infectious Diseases.
We will achieve new breakthroughs in science and medicine and I see what they’re doing. I see it. They show me the things we’re doing in our country today. There’s never been anything like it. We will be ending the AIDS epidemic shortly in America.
President Trump at a speech Aug 1 2019 in Cincinnati, Ohio [source]
What are broadly neutralizing antibodies?
In 2016 NIH scientists discovered the N6 antibody in blood samples collected from an HIV-resistant patient.
Scientists from the National Institutes of Health have identified an antibody from an HIV-infected person that potently neutralized 98 percent of HIV isolates tested, including 16 of 20 strains resistant to other antibodies of the same class. The remarkable breadth and potency of this antibody, named N6, make it an attractive candidate for further development to potentially treat or prevent HIV infection, say the researchers.
"NIH Scientists Identify Potent Antibody that Neutralizes Nearly All HIV Strains." NIH Newsroom. [source]
Humans naturally develop antibodies as a response to disease. These antibodies selectively bind to and disarm pathogens like HIV. Your natural immunity is made possible by antibodies.
Broadly neutralizing antibodies (bNAbs) are rare. It takes three to three to five years of chronic stimulation by a virus before the immune system can generate potent and broadly cross-reactive antibody. And even then, most people still cannot make them.
Minicircle.HIV is a project to develop a safe, reliable means to share the genetic information encoding immunity from person to person.
Why is HIV so hard to cure?
HIV is coated with bulky sugar molecules that themselves are not immunogenic and largely deflect immune responses mounted against the virus.
“Antibodies prefer to see proteins. It’s very hard for an antibody to navigate that glycan shield, find a protein, and neutralize.”
John Mascola, director of the Vaccine Research Center (VRC) at the U.S. National Institute of Allergy and Infectious Diseases.
Despite its glycan shield, HIV’s outer protein known as Envelope (Env) actually has many sites of vulnerability.
“Virtually the entire Env can be targeted [by antibodies]."
Andrew Ward, a professor at Scripps Research in La Jolla, CA, and a principal investigator of IAVI’s Neutralizing Antibody Center (NAC).
Another reason HIV is hard to cure is because of its latent reservoir. HIV infects CD4 white blood cells by penetrating the cell surface and injecting its genome. This genome contains instructions for editing the host cell's genome to include HIV production.
Even if all HIV particles can be removed from the blood using traditional anti-retroviral therapy or broadly neutralizing antibodies, the CD4 cells themselves are still infected, and would either need to be removed, or gene edited.
The process of removing the latent reservoir has only been documented in humans once. Administration of the antibody Nivolumab to an HIV-infected patient in France in 2017 showed for the first time a dumping of the latent reservoir.
This is the first demonstration of this mechanism working in humans. It could have implications for HIV patients, both with and without cancer, as it can work on HIV reservoirs and tumor cells independently. The absence of side effects in this patient is also good news, and suggests this could be an optimum treatment for HIV-infected patients with cancer.
Jean-Philippe Spano, MD, PhD, head of medical oncology at Pitie-Salpetriere Hospital AP-HP in Paris
A fourth reason curing HIV is so difficult is because of gp120. Even if all HIV particles are removed, and the latent reservoir dumped, gp120 will still be present in all bodily tissues. gp120 is a neurotoxin and prevents stem cells in the brain from producing new brain cells. gp120 is difficult for the body to get rid of and been proven to aide infection by inducing apoptosis (cell death), mitochondrial death, and oxidative stress. [source]
The production of unnecessarily large amounts of intracellular and extracellular protein aggregates is associated with other diseases, like Alzheimer's and SENS Research Foundation has defined it as a hallmark of aging.
A fourth reason why curing HIV is so difficult is because of neuro-AIDS, also called HIV-associated neurocognitive disorder (HANDS). HIV infects the brain almost immediately upon arrival to the body, and causes brain cells to secrete neurotoxins, causing brain death. Because of the blood-brain barrier (BBB), most antibodies cannot pass into the brain. Meaning it is very unlikely that any vaccine ever produced could end neuro-AIDS for someone already infected. To make matters worse, anti-retroviral drugs like efavirenz are toxic to the central nervous system. This may be behind the cause of why the drug causes intense dreaming and is used for recreational purposes. [source]
A New Hope
After decades of disappointing results from clinical trials, there are now more funded clinical trials than ever searching for a cure. Many of the traditional vaccine approaches will not work, and trials using old antibodies like VRC01 can also not be expected to work.
However, new research with broadly neutralizing antibodies suggests a cure may be found.
- Dr. Desrosiers’s team at the University of Miami in 2014 used a viral vector to transfer two bNAb genes into monkeys which resulted in a durable suppression of SHIV, a close relative to HIV in simians.[source]
- Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center at Harvard, presented data at HIVR4P showing that the combination of bNAbs PGT121 and GS-9620 completely protects rhesus macaques from 12 SHIV challenges. 16-weeks of administration cured 6 of 11 monkeys of SHIV with no viral rebound after 140 days. [source] [source]
- Researchers in Hong Kong in 2018 tested BilA-SG. An infusion of this single antibody was enough to prevent transmission of HIV to HIV humanized mice. [source]
“When it comes to getting a good clinical agent, we’re going to need a combination."
Bette Korber, computational biologist at Los Alamos National Laboratory.
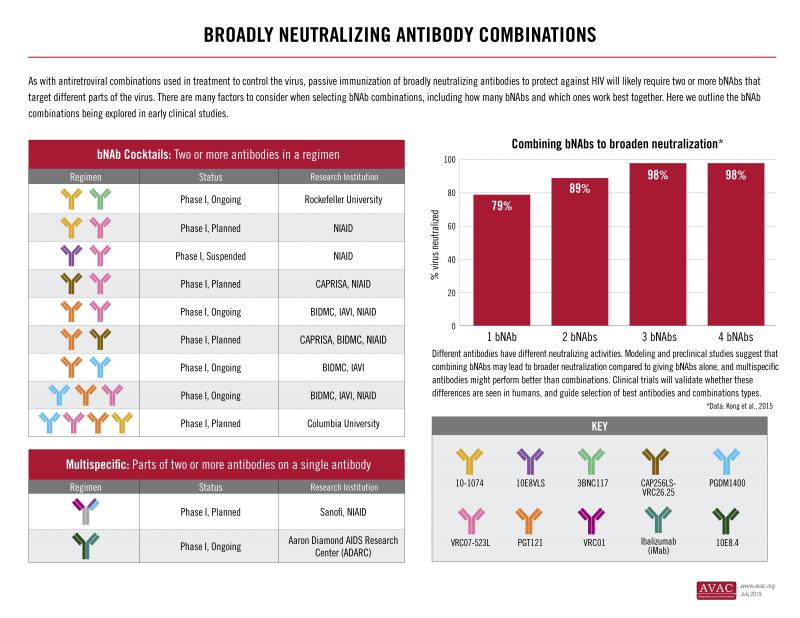
Because HIV is a diverse and rapidly mutating virus, combinations of antibodies working in tandem are necessary in order to create a vaccine that would truly neutralize all strains.
One interesting development in HIV bNAbs science is the development of multivalent antibodies – synthetic antibodies which combine the best attributes of multiple antibodies into one single antibody. Multivalent antibodies are significantly more potent.
Of the multivalent antibodies, 10E8v4-5R+100cF is one of the best. It neutralizes HIV particles from every clade and leaves almost none to spare.
However, the 10E8 backbone has been shown in human studies to produce a poorly understood inflammatory response, something very rare for antibodies.

